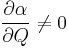Raman scattering
Raman scattering or the Raman effect ( /ˈrɑːmən/) is the inelastic scattering of a photon. It was discovered by Sir Chandrasekhara Venkata Raman and Kariamanickam Srinivasa Krishnan in liquids,[1] and by Grigory Landsberg and Leonid Mandelstam in crystals.[2][3]
When photons are scattered from an atom or molecule, most photons are elastically scattered (Rayleigh scattering), such that the scattered photons have the same kinetic energy (frequency) and wavelength as the incident photons. However, a small fraction of the scattered photons (approximately 1 in 10 million photons) is scattered by an excitation, with the scattered photons having a frequency different from, and usually lower than, the frequency of the incident photons.[4] In a gas, Raman scattering can occur with a change in transitional energy of a molecule (see energy level). Chemists are concerned primarily with the transitional Raman effect.
The inelastic scattering of light was predicted by Adolf Smekal in 1923[5] (and in German-language literature it may be referred to as the Smekal-Raman effect [6]). In 1922, Indian physicist C. V. Raman published his work on the "Molecular Diffraction of Light," the first of a series of investigations with his collaborators that ultimately led to his discovery (on 28 February 1928) of the radiation effect that bears his name. The Raman effect was first reported by C. V. Raman and K. S. Krishnan, and independently by Grigory Landsberg and Leonid Mandelstam, in 1928. Raman received the Nobel Prize in 1930 for his work on the scattering of light. In 1998[7] the Raman effect was designated an ACS National Historical Chemical Landmark in recognition of its significance as a tool for analyzing the composition of liquids, gases, and solids.[8]
Contents |
Stokes and anti-Stokes scattering
There are two types of Raman scattering, Stokes scattering and anti-Stokes scattering.
The interaction of light with matter in a linear regime allows the absorption and emission of a photon precisely matching the difference in energy levels of the interacting electron or electrons.
The Raman effect corresponds, in perturbation theory, to the absorption and subsequent emission of a photon via an intermediate vibrational state, having a virtual energy level (see also: Feynman diagram). There are three possibilities:
- no energy exchange between the incident photons and the molecules (and hence no Raman effect)
- energy exchanges occur between the incident photons and the molecules. The energy differences are equal to the differences of the vibrational and rotational energy-levels of the molecule. In crystals only specific phonons are allowed (solutions, which do not cancel themselves, of the wave equations) by the periodic structure, so Raman scattering can only appear at certain frequencies. In amorphous materials like glasses, more phonons are allowed and thereby the discrete spectral lines become broad.
-
- molecule absorbs energy: Stokes scattering. The resulting photon of lower energy generates a Stokes line on the red side of the incident spectrum.
- molecule loses energy: anti-Stokes scattering. Incident photons are shifted to the blue side of the spectrum, thus generating an anti-Stokes line.
These differences in energy are measured by subtracting the energy of the mono-energetic laser light from the energy of the scattered photons. The absolute value, however, doesn't depend on the process (Stokes or anti-Stokes scattering), because only the energy of the different vibrational levels is of importance. Therefore, the Raman spectrum is symmetric relative to the Rayleigh band. In addition, the intensities of the Raman bands are only dependent on the number of molecules occupying the different vibrational states, when the process began. If the sample is in thermal equilibrium, the relative numbers of molecules in states of different energy will be given by the Boltzmann distribution:
|
|
where: |
|
Thus lower energy states will have more molecules in them than will higher (excited) energy states. Therefore, the Stokes spectrum will be more intense than the anti-Stokes spectrum.
Distinction from fluorescence
The Raman effect differs from the process of fluorescence. For the latter, the incident light is completely absorbed and the system is transferred to an excited state from which it can go to various lower states only after a certain resonance lifetime. The result of both processes is in essence the same: A photon with the frequency different from that of the incident photon is produced and the molecule is brought to a higher or lower energy level. But the major difference is that the Raman effect can take place for any frequency of the incident light. In contrast to the fluorescence effect, the Raman effect is therefore not a resonant effect. In practice, this means that a fluorescence peak is anchored at a specific excitation frequency, whereas a Raman peak maintains a constant separation from the excitation frequency.
Selection rules
The distortion of a molecule in an electric field, and therefore the vibrational Raman cross section, is determined by its polarizability.
A Raman transition from one state to another, and therefore a Raman shift, can be activated optically only in the presence of non-zero polarizability derivative with respect to the normal coordinate (that is, the vibration or rotation):
Raman-active vibrations/rotations can be identified by using almost any textbook that treats quantum mechanics or group theory for chemistry. Then, Raman-active modes can be found for molecules or crystals that show symmetry by using the appropriate character table for that symmetry group.
Stimulated scattering and amplification
Raman amplification can be obtained by using stimulated Raman scattering (SRS), which actually is a combination of a Raman process with stimulated emission. It is interesting for application in telecommunication fibers to amplify inside the standard material with low noise for the amplification process. However, the process requires significant power and thus imposes more stringent limits on the material. The amplification band can be up to 100 nm broad, depending on the availability of allowed photon states.
SRS is one of the processes that impede the laser coupling of inertial confinement fusion capsules.
Spectrum generation
For high-intensity CW (continuous wave) lasers, SRS can be used to produce broad bandwidth spectra. This process can also be seen as a special case of four-wave mixing, wherein the frequencies of the two incident photons are equal and the emitted spectra are found in two bands separated from the incident light by the phonon energies. The initial Raman spectrum is built up with spontaneous emission and is amplified later on. At high pumping levels in long fibers, higher-order Raman spectra can be generated by using the Raman spectrum as a new starting point, thereby building a chain of new spectra with decreasing amplitude. The disadvantage of intrinsic noise due to the initial spontaneous process can be overcome by seeding a spectrum at the beginning, or even using a feedback loop as in a resonator to stabilize the process. Since this technology easily fits into the fast evolving fiber laser field and there is demand for transversal coherent high-intensity light sources (i.e., broadband telecommunication, imaging applications), Raman amplification and spectrum generation might be widely used in the near-future.
Applications
Raman spectroscopy employs the Raman effect for materials analysis. The spectrum of the Raman-scattered light depends on the molecular constituents present and their state, allowing the spectrum to be used for material identification and analysis. Raman spectroscopy is used to analyze a wide range of materials, including gases, liquids, and solids. Highly complex materials such as biological organisms and human tissue[9] can also be analyzed by Raman spectroscopy.
For solid materials, Raman scattering is used as a tool to detect high-frequency phonon and magnon excitations.
Raman lidar is used in atmospheric physics to measure the atmospheric extinction coefficient and the water vapour vertical distribution.
Stimulated Raman transitions are also widely used for manipulating a trapped ion's energy levels, and thus basis qubit states.
Raman amplification is used in optical amplifiers.
See also
- Scattering
- Brillouin scattering
- Nonlinear optics
- Fiber amplifier
- List of surface analysis methods
- Raman laser
- Raman spectroscopy
- Surface Enhanced Raman Spectroscopy (SERS)
- Inverse Raman effect
- Resonance Raman spectroscopy (RR)
- Coherent anti-Stokes Raman spectroscopy (CARS)
- Depolarization ratio
Notes
- ^ Singh, R. (2002). "C. V. Raman and the Discovery of the Raman Effect". Physics in Perspective (PIP) 4 (4): 399–420. Bibcode 2002PhP.....4..399S. doi:10.1007/s000160200002.
- ^ Landsberg, G.; Mandelstam, L. (1928). "Eine neue Erscheinung bei der Lichtzerstreuung in Krystallen". Naturwissenschaften 16 (28): 557. Bibcode 1928NW.....16..557.. doi:10.1007/BF01506807.
- ^ Smekal, A. (1923). "Zur Quantentheorie der Dispersion". Naturwissenschaften 11 (43): 873–875. Bibcode 1923NW.....11..873S. doi:10.1007/BF01576902.
- ^ Harris and Bertolucci (1989). Symmetry and Spectroscopy. Dover Publications. ISBN 048666144X.
- ^ <A. Smekal: Zur Quantentheorie der Dispersion. In: Die Naturwissenschaften. 11, Nr. 43, 1923, S. 873-875, doi:10.1007/BF01576902.
- ^ Nature (1931-12-19). "A review of the 1931 book ''Der Smekal-Raman effekt''". Nature.com. http://www.nature.com/nature/journal/v128/n3242/abs/1281026c0.html. Retrieved 2011-09-17.
- ^ "Raman effect". Portal.acs.org. http://portal.acs.org/portal/acs/corg/content?_nfpb=true&_pageLabel=PP_ARTICLEMAIN&node_id=924&content_id=WPCP_007605&use_sec=true&sec_url_var=region1. Retrieved 2011-09-17.
- ^ "Frontiers of Knowledge, ACS web". Acswebcontent.acs.org. 2006-10-09. http://acswebcontent.acs.org/landmarks/front_t2.html#Raman. Retrieved 2011-09-17.
- ^ "Painless laser device could spot early signs of disease". BBC News. 27 September 2010. http://www.bbc.co.uk/news/science-environment-11390951.
References
- "A new radiation", Indian J. Phys., 2 (1928) 387 - http://www.uky.edu/~holler/raman.html
- Herzberg, Spectra of Diatomic Molecules, Litton Educational Publishing, 1950, ISBN 0-442-03385-0, pp. 61ff and 66ff
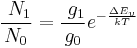
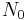 : number of atoms in the lower vibrational state
: number of atoms in the lower vibrational state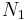 : number of atoms in the higher vibrational state
: number of atoms in the higher vibrational state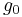 :
: 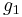 : degeneracy of the higher vibrational state
: degeneracy of the higher vibrational state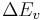 : energy difference between these two vibrational states
: energy difference between these two vibrational states